It’s an unfortunate fact that 1 in 2 people in the UK will be diagnosed with cancer in their lifetime. However, it is well-known that usually the earlier the diagnosis, the better the outcome. Therefore, as well as searching for new treatments, cancer research has focused on finding new ways to detect cancer quickly and easily at the earliest stage possible to improve survival rates.
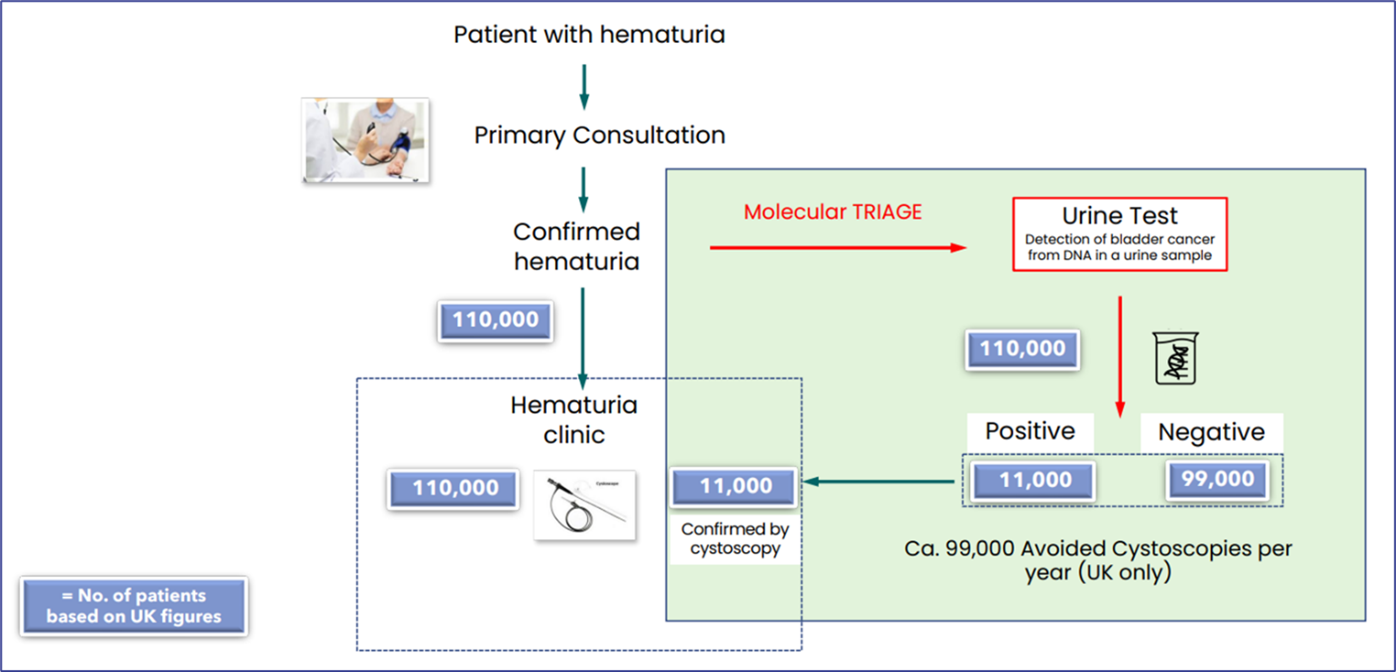
Currently, people with blood in the urine (haematuria) and suspected of having bladder cancer are referred for a cystoscopy - an uncomfortable hospital procedure that involves a camera being passed through the urethra into the bladder. A genetic test for identifying all stages of bladder cancer from a simple urine sample is an effective, non-invasive method with equivalent sensitivity and specificity to a cystoscopy.
This 'liquid biopsy' technology sequences DNA in the urine to identify mutations in 23 different genes which are found in 96% of all bladder cancers. The ease of providing a urine sample in the comfort of your own home over a cystoscopy procedure in hospital is clearly advantageous. Not only does this make it highly accessible for those experiencing haematuria with an unknown cause, but as bladder cancer has one of the highest reoccurrence rates of any cancer, it is also beneficial for relapse surveillance.
This pioneering non-invasive accurate genetic test for bladder cancer from a home urine sample has 85% specificity and up to 90% sensitivity and now available to BUPA bladder cancer policy holders.
Have you been diagnosed with blood in the urine (haematuria) and awaiting a cystoscopy to rule out cancer as the cause? If so, the GALEAS™ Bladder Cancer Test could be for you. It is a molecular biomarker test that can detect genetic mutations associated with 96% of bladder cancers at all stages and grades, with equivalent results to cystoscopy. It provides a much more convenient way of sampling which can also exclude the diagnosis altogether, and removes the need for your cystoscopy!
Whilst other urine tests for bladder cancer are available, they are often based on the levels of proteins or RNA in the urine. These are not specific, nor causally linked, to bladder cancer and therefore lack the specificity and sensitivity to detect small or low-grade tumours. Unlike other tests, this GALEAS™ Bladder Cancer Test also covers a wider range of DNA markers, so it is less likely to miss a cancer.
Early diagnosis of the disease is crucial with more than 80% of people diagnosed at the earliest stage surviving at least five years compared to less than 10% diagnosed at the latest stage.
Please note that this test is NOT suitable for everyone. It is a clinically approved test only for individuals who have a confirmed diagnosis of blood in the urine.
This non-invasive pain-free urine test is simple, easy, and can be done at home with no appointment needed. After sending away your sample, the laboratory will analyse your urine and generate a clear and understandable report of the findings. This is then emailed directly to you, and the results can be discussed further with your doctor if needed. It is also able to streamline diagnosis of different types of bladder cancer, non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC).
The GALEAS™ Bladder Cancer Test analyses the DNA in your urine for the presence of mutations associated with all types of bladder cancer and results are shared directly with you.

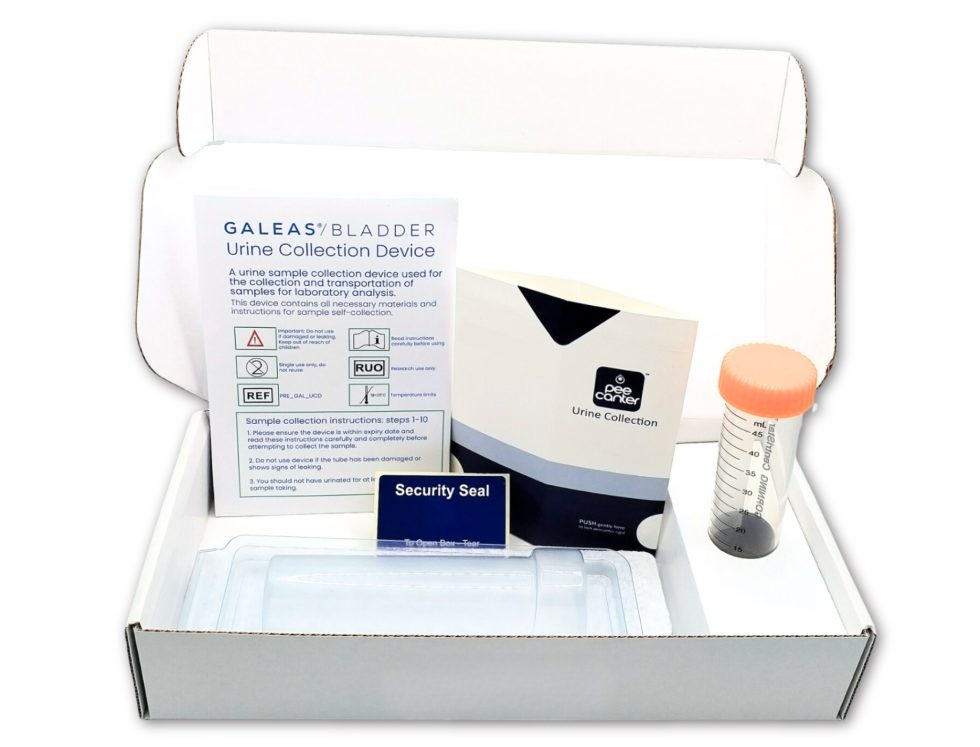
Order online and we will send you a urine sampling kit containing everything you need.
Collect your first urine sample of the day, straight after waking, carefully following the instructions provided. Return the sample in the pre-paid Tracked 48 packaging.

DNA is extracted from your urine sample, sequenced, analysed and reported back to you within 15 working days.

Results are sent via email directly to you. If a mutation associated with bladder cancer is detected you will be advised to contact your doctor immediately.
Your kit will include easy to follow instructions for use plus the following items:
A) Urine sample collection device (peecanter) and tube (50ml required)
B) Protective transport mailer for urine tube
C) Pack Contents/Instructions For Use card
D) Galeas Bladder Cancer Test Consent Form
E) Tamperproof sticker
F) Pre-paid postage return box
Please ensure you fill the collection device with your first pass urine of the day. This sample will be the most concentrated with the cells requrired for the test.


There are two possible outcomes:
Bladder cancer variants not detected (~90% of cases);
No evidence of cancer-associated somatic genomic variants detected suggesting the presence of bladder cancer is unlikely.
Bladder cancer variants detected (~10% of cases);
Evidence of cancer-associated somatic genomic variants detected suggesting there is high likelihood that cancer is present. Appropriate clinical recommendations will be made. You will be advised that it is essential to follow up with your doctor immediately as currently the definitive diagnosis of bladder cancer is confirmed with a cystoscopy.
